Content
What is the Current Bioplastics in Diagnostic Devices Market Size and Share?
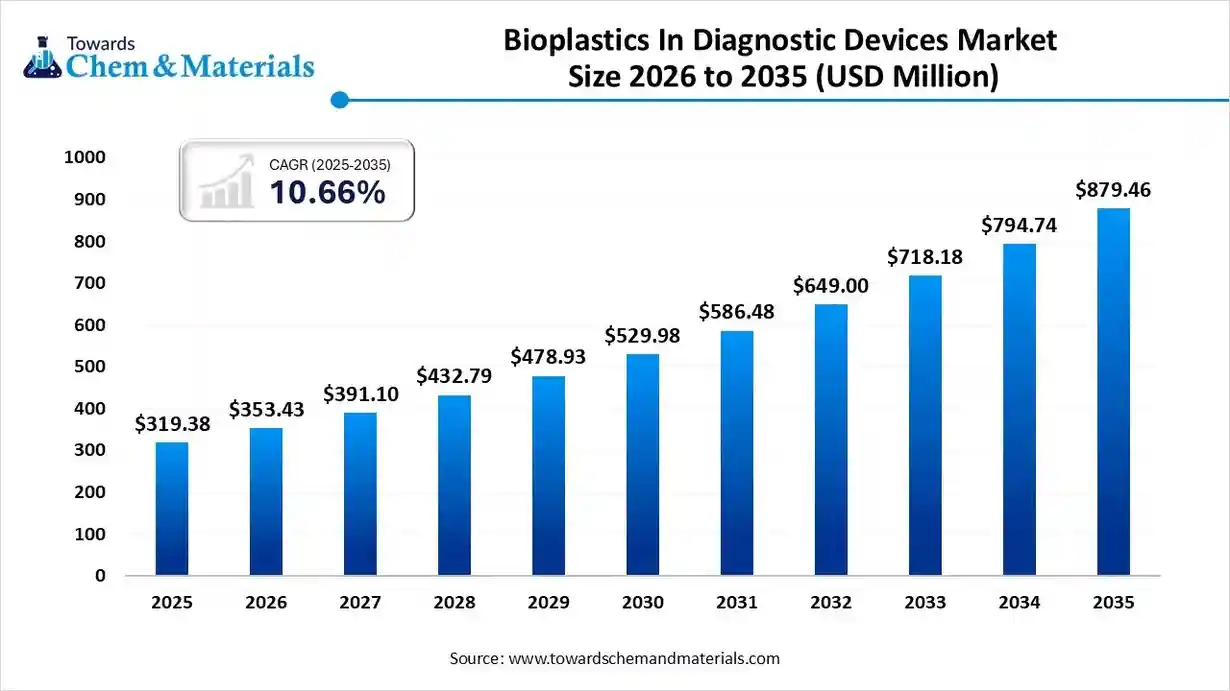
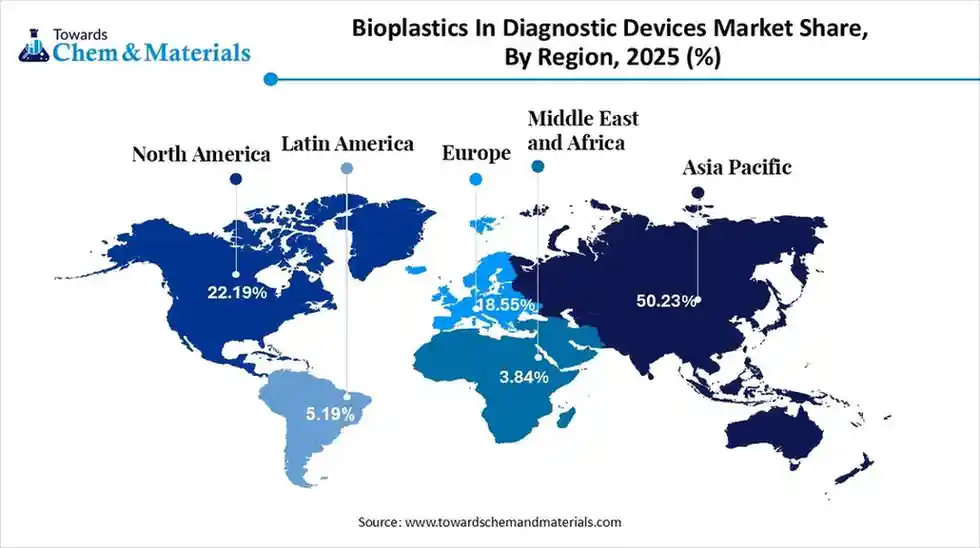
The global bioplastics in diagnostic devices market size was estimated at USD 319.38 million in 2025 and is predicted to increase from USD 353.43 million in 2026 and is projected to reach around USD 879.46 million by 2035, The market is expanding at a CAGR of 10.66% between 2026 and 2035. Asia Pacific dominated the bioplastics in diagnostic devices market with a market share of 50.23% the global market in 2025. The shift towards eco-friendly manufacturing and biodegradable materials has contributed to favorable market economics for the industry in recent years.
Key Takeaways
- By region, Asia Pacific led the bioplastics in diagnostic devices market with the largest revenue share of over 50.23% in 2025, owing to the presence of advanced manufacturing and greater medical device component export in the current period.
- By material type, the PHA segment led the market with the largest revenue share of 51.87% in 2025, akin to its super biodegradability and biological compatibility.
- By application type, the rapid test kits segment led the market with the largest revenue share of 62.05% in 2025, akin to their use everywhere, like homes, clinics, airports, and hospitals.
Safe, Stable, and Sustainable: The Rise of Bioplastics in Diagnostics
The bioplastic in diagnostic devices refers to the biodegradable plastic materials, which are specifically designed with an understanding of the importance of medical safety for the medical testing tools, such as sample tubes, test cartridges, and disposable diagnostic kits. Moreover, having characteristics like chemical stability in the single-use medical applications, the medical bioplastics have presented new business models for forward-thinking manufacturers in recent years.
Bioplastics in Diagnostic Devices Market Trends:
- The increased demand and the usage of the disposable point-of-care diagnostic devices have supported the industry's potential in the past few years. Moreover, the consumers are turning towards the blood markers, rapid tests for infections, and home diagnostics, which are primarily made for single use, as per the observation.
- The increased shift towards the bioplastic, which has the chemical compatibility with material transference, may create contract manufacturing and OEM opportunities in the upcoming years. Also, having the requirement of transparent material in devices, which is indicated by changing colours and an alarming sensor, is likely to enable the sector to explore untapped potential in the coming period.
- The emergence of the lightweight and transport-friendly diagnostic kits has allowed the stakeholders to capitalize on growth opportunities in recent years. Also, having the minimum weight and easy-to-mold properties, the bioplastics have actively enhanced the market readiness and future industry capabilities in the past few years, as per the recent observations.
Report Scope
| Report Attribute | Details |
| Market Size Value in 2026 | USD 353.43 Million |
| Revenue Forecast in 2035 | USD 879.46 Million |
| Growth Rate | CAGR 10.66% |
| Forecast Period | 2026 - 2035 |
| Base Year | 2025 |
| Dominant Region | Asia Pacific |
| Fastest Growing Region | North America |
| Segment Covered | By Material, By Application, By Region |
| Key companies profiled | Danimer Scientific, Corbion, NatureWorks, BASF SE, Mitsubishi Chemical, Kaneka Corporation, Novamont, FKuR Kunststoff GmbH |
From Green Material to Precision Tools: Medical Bioplastic Evolves
The move toward purpose-built medical bioplastics instead of general eco-plastics is likely to create significant opportunities during the projected period. Earlier, bioplastics were mainly used to reduce environmental impact. Now, materials are being designed to control fluid movement, reagent stability, and sensor accuracy inside diagnostic devices.
Value Chain Analysis of the Bioplastics in Diagnostic Devices Market:
- Distribution to Industrial Users: Bioplastics are distributed to industrial users in the diagnostic devices market primarily through direct sales, specialized distributors, and converters who partner with medical device manufacturers (OEMs). The primary industrial users are the medical device manufacturers themselves, with key material suppliers dominating the market.
- Key Players: TotalEnergies Corbion, and NatureWorks LLC
- Chemical Synthesis and Processing :The chemical synthesis of bioplastics in diagnostic devices market involves two primary methods: chemical polymerization for materials like polylactic acid (PLA), and microbial fermentation for polyhydroxyalkanoates (PHAs). The resulting polymers are then processed using standard techniques like injection molding and extrusion to form final products.
- Key Players: BASF SE and Danimer Scientific
- Regulatory Compliance and Safety Monitoring: Regulatory compliance for bioplastics in diagnostic devices market is governed by rigorous, globally recognized standards focused primarily on biocompatibility, safety, and quality management. Manufacturers must adhere to specific national and international regulations, such as those set by the FDA and through the EU MDR, with ISO 10993 as the central guidance for biological evaluation.
- Key Agencies: REACH (Registration, Evaluation, Authorisation, and Restriction of Chemicals) and Environmental Protection Agency
Bioplastics in Diagnostic Devices Market Regulatory Landscape: Global Regulations
| Country / Region | Regulatory Body | Key Regulations | Focus Areas |
| United States | Food and Drug Administration (FDA) | Federal Food, Drug, and Cosmetic Act (FD and C Act) | Ensuring device safety and efficacy |
| European Union | European Commission (EC) | Medical Devices Regulation (MDR) (EU) 2017/745 | Rigorous pre-market assessment to meet MDR/IVDR requirements |
| China | National Medical Products Administration (NMPA) | Regulations on the Supervision and Administration of Medical Devices | Ensuring device safety and efficacy to meet NMPA approval |
Segmental Insights
Material Type Insights
How did the PLA Segment Dominate Bioplastics in Diagnostic Devices Market in 2025?
The PLA segment dominated the market in 2025, akin to factors like easier processing, wide availability, and recent adoption in medical applications. Moreover, by allowing manufacturers to mold precisely according to the shapes, the PLA segment has gained major industry attention in recent years. Also, factors like better rigidity and clarity have positively impacted revenue potential and industry scalability in recent years.
By material type, the PHA segment led the market with the largest revenue share of 51.87% in 2025. The PHA segment is expected to grow with a rapid CAGR, due to its super biodegradability and biological compatibility. Furthermore, the increasing global government support for recycling initiatives with single-use plastic bans has contributed to the growth of the segment in the past few years. Also, the PHA has been seen performing better with biological fluids and reagents in recent years.
Application Type Insights
Why does the Rapid Test Kits Segment Dominate the Bioplastics in Diagnostic Devices Market?
By application type, the rapid test kits segment led the market with the largest revenue share of 62.05% in 2025, The rapid test kits segment dominated the market in 2025, due to their use everywhere, like homes, clinics, airports, and hospitals. Most of these kits are thrown away after one use, so companies are choosing bioplastics to reduce waste problems. Bioplastics are safe, clean, and easy to shape, which makes them perfect for test kit shells. During health testing drives, millions of kits are produced in a short time, and PLA bioplastics help manufacturers keep costs low and production fast.
The cartridges and microfluidic consumables segment is expected to grow at a rapid CAGR akin to modern diagnostic machines use them for every test. These small plastic parts control how blood or samples move inside machines and are thrown away after one use. Bioplastics work well because they can be molded very accurately and are better for waste handling. As testing becomes more automated, each test needs a new cartridge, increasing demand.
Regional Insights
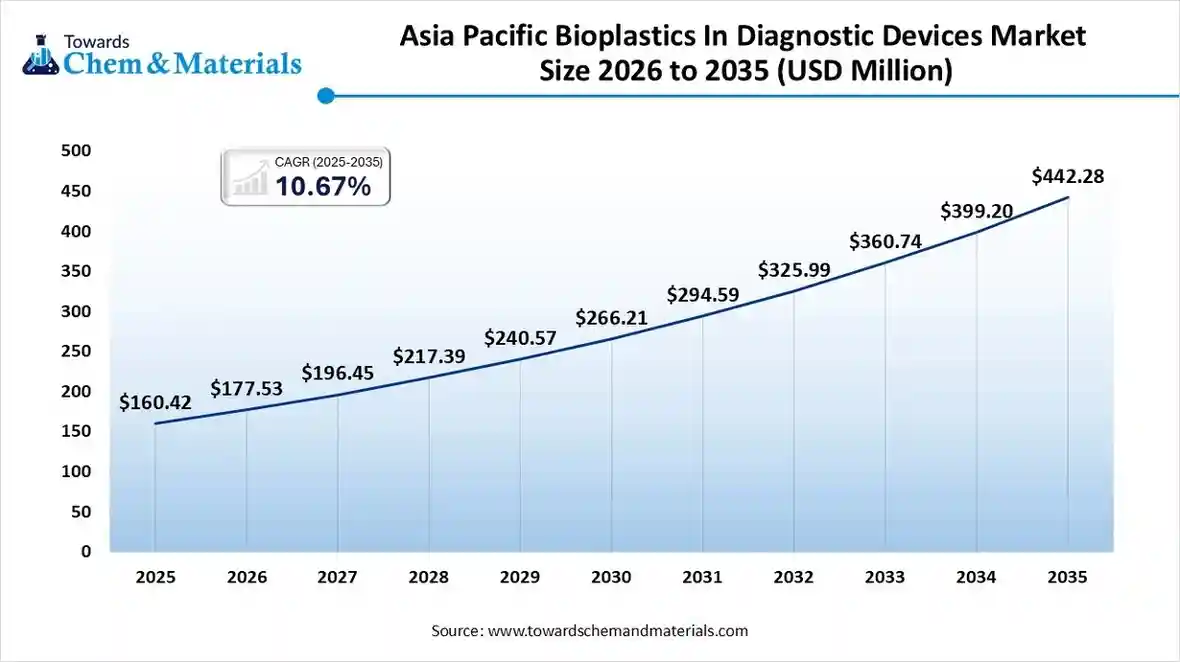
The Asia Pacific bioplastics in diagnostic devices market size was valued at USD 160.42 million in 2025 and is expected to reach USD 442.28 million by 2035, growing at a CAGR of 10.67% from 2026 to 2035. Asia Pacific dominated the bioplastics in diagnostic devices market in 2025, akin to the presence of advanced manufacturing and greater medical device component export in the current period. Also, the advantages like low-cost production, skilled workforce, and advanced technology access have created lucrative opportunities in the region nowadays.
China Reinforces Leadership in Bioplastic Diagnostic Device Manufacturing
China maintained its dominance in the market, akin to the heavy sustainability implementation in the manufacturing field and adoption of smart technology. Moreover, China has recently seen the production of rapid test kits in huge volumes and diagnostic consumables, which support the capital growth and economic activity in the sector.
North America Bioplastics in Diagnostic Devices Market Examination
North America is expected to capture a major share of the market with a rapid CAGR, due to the increased regional focus towards precision medicine and domestic manufacturing in recent years. Moreover, the region has been seen under a demand for high-value cartridges, which require specialized materials in the coming years.
Advanced R&D Positions the United States for Long-Term Bioplastics Expansion
The United States is expected to emerge as a prominent country for the bioplastics in diagnostic devices market in the coming years, due to the increasing shift towards environmental regulations and hospital sustainability in recent years. Moreover, the greater R&D infrastructure and material science technology have positioned the industry for long-term expansion in the current period.
Europe Bioplastics in Diagnostic Devices Market Evaluation
Europe expects a notable growth in the market. The region’s advanced diagnostic infrastructure and its needs, and engineering expertise are poised to enhance market participation for producers in the upcoming years. Also, the major brands in Europe have been seen under the heavy replacement of petroleum plastic with PLA and PHA material, as per the survey.
Regulatory Pressure Accelerates Bioplastic Adoption in Germany
Germany is expected to gain a major industry, owing to major German manufacturers are focused on high-quality diagnostic devices manufacturing and high precision nowadays. Moreover, rules released by the European Union have put pressure on plastic manufacturers to shift to eco-friendly manufacturing, which is actively creating lucrative opportunities in the country for the current period.
Bioplastics in Diagnostic Devices Market Study in the Middle East and Africa
The Middle East and Africa are expected to capture a notable share of the industry. As healthcare testing is increasing in the Middle East & Africa, with is a need to manage medical waste better in recent years. Moreover, many countries are building new labs and diagnostic centers, which use large numbers of disposable test items.
Healthcare Investments Drive Bioplastic Adoption in Saudi Diagnostics
Saudi Arabia is expected to emerge as a prominent country for bioplastics in the diagnostic devices market in the coming years due to the country is investing heavily in healthcare, which means more testing and more disposable diagnostic tools. Managing medical waste is becoming important. Bioplastics are gaining attention in the region nowadays. These materials allow safe testing while supporting cleaner disposal. New hospitals and labs prefer modern materials that align with sustainability goals in the region nowadays.
South America Bioplastics in Diagnostic Devices Market Evaluation
South America is a notably growing region, as diagnostic testing expands in public and private healthcare systems. Countries are improving disease screening and access to rapid testing. As South America has seen, under the routine health checks and infectious diseases have surged in recent years. This creates large volumes of disposable test items. Bioplastics help reduce the environmental burden of this waste.
Sustainable Diagnostics Take Shape as Brazil Advances Bioplastics Adoption
Brazil is expected to gain a major industry due to the local producers exploring bioplastics to improve sustainability while keeping costs manageable. This practical need for cleaner testing solutions is driving the steady growth of bioplastics in diagnostic devices across South America.
Recent Developments
- In November 2025, Elm-plastic GmbH achieved a milestone in the medical bioplastic industry by introducing the bioplastic pipette. Also, this is the world's first bioplastic pipette, which is specifically designed for pharmaceutical and medical usage, as per the company's claim.(Source: www.plasticsnews.com)
Top Vendors in the Bioplastics in Diagnostic Devices Market and Their Offerings:
- NatureWorks: A leading producer of Ingeo bio-polymer (polylactic acid or PLA) from renewable resources, used in a wide range of applications from packaging and food service ware to textiles and industrial parts.
- TotalEnergies Corbion PLA: A global leader in the production and marketing of Luminy PLA (polylactic acid) bioplastics, offering a wide range of PLLA and PDLA grades for use in food packaging, durable products, and fiber.
- Corbion: A global market leader in lactic acid and lactic acid derivatives, providing high-performance biochemicals and sustainable food solutions, and a key partner in the TotalEnergies Corbion PLA joint venture.
- Danimer Scientific: An American company specializing in the creation of biodegradable biopolymers known as Nodax PHA (polyhydroxyalkanoates), which can replace many conventional petroleum-based plastics in various single-use applications.
Top Companies in the Bioplastics in Diagnostic Devices Market

- Danimer Scientific
- Corbion
- TotalEnergies Corbion PLA
- NatureWorks
- BASF SE
- Mitsubishi Chemical
- Kaneka Corporation
- Novamont
- FKuR Kunststoff GmbH
Segments Covered in the Report
By Material
- PLA
- PHA
- PBS/PBAT
- Other Materials
By Application
- Rapid Test Kits
- Cartridges & Microfluidic Consumables
- Instrument Housings
- Other Applications
By Region
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Asia Pacific
- China
- India
- Japan
- South Korea
- Latin America
- Brazil
- Argentina
- Middle East & Africa
- Saudi Arabia
- South Africa
